The temperature
Heat and temperature are familiar concepts, which are commonly used as synonyms to describe the feeling of hot and cold procured by a body. The distinction between a hot and a cold body is linked to the sensation given by the contact with the body so, it is a relative concept. However, heat and temperature are two well-defined properties or physical sizes so, they can be measurable and they can embody two distinct physical concepts, even though both are closely related to the intimate structure of matter. The molecule is the smallest part of the substance that has all the characteristics of the substances themselves. They are composed of smaller particles called “atoms”. If a substance is composed of a single type of atom it is called “element”, if it has more types of atoms it is called “compound”. It is made up of smaller particles “the nucleus” and a certain number of “electrons” that turn around it, not all the atoms have the same number of electrons and not all the electrons go around the nucleus at the same distance. Temperature is an index of the state of thermal agitation of electrons, the greater is the thermal agitation, the higher is the temperature of a substance. To measure the temperature, we use the thermometer, it consists of a glass tube with a thin tube inside which contains mercury. When it gets in contact with a hot body both the glass and the mercury heat up and mercury arises for expansion where there is a graduate scale. There are 3 types of temperature scales, they are:
1) the Celsius scale (Celsius or Centigrade). The Celsius scale, was introduced by 'mathematical Swedish astronomer Anders Celsius (1701-1744). In this scale the value 0 is assigned at the temperature of melting ice and a value of 100 at the temperature of boiling water.
2) the Kelvin scale (Kelvin). In the SI they use the Kelvin scale introduced in 1847by the Scottish physicist William Thomas (1824-1907) called Lord Kelvin. In it a value of 273.15 is assigned at the temperature of melting ice and the value 373 at the temperature of 'hot water. Also the interval between these two temperatures is divided into 100 equal parts and each part is called Kelvin degree (K). To switch between a temperature in Celsius degrees (Tc) to a given in Kelvin (TK) using the equation: Tk = Tc +273.15
3) the Fahrenheit scale (Fahrenheit). In Anglo-Saxon countries they use the Fahrenheit scale introduced by the scientist Daniel Gabriel Fahrenheit (1686-1736). In this scale the value 32 is assigned to the temperature of the ice while the value 212 at the boiling point both at atmospheric pressure at sea level . There is therefore a division into 180 equal parts, each representing one degree Fahrenheit (symbol F) ° To go from one temperature in degrees Celsius (Tc) to a given in Fahrenheit (F) using the equation: F = 32 +1.8 * Tc
To convert degrees from Celsius to Fahrenheit and vice versa click here
.
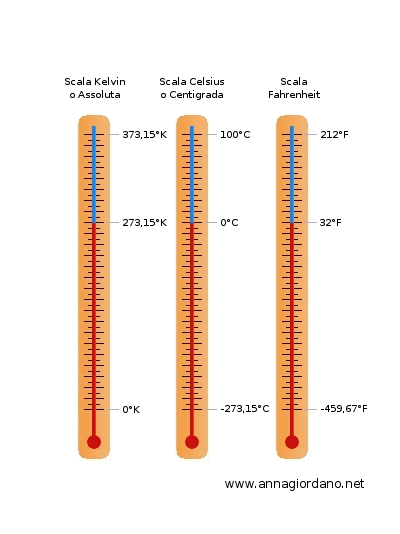
Termometro a mercurio con scala Kelvin, Celsius e Fahrenheit
In Italian
|